BLUE Software Brand Consistency and Management Software for Development of Cosmetic Products


BLUE Software is a provider of management solutions for brand lifecycles to boost the worldwide profile of products in the consumer, retail, and life sciences markets. The software helps achieve integration with business processes for brand delivery and compliance throughout the media on a global-scale.
More than 100,000 users and 3,500 firms have access to the enterprise-level software-as-a service (SaaS) platform BLUE, taking advantage of functions that include automated workflow, digital library of assets library, as well as customisable modules.
Robust e-reporting systems for commercial product development
BLUE’s full power is achieved through comprehensive e-reporting capabilities. Key Performance Indicator KPI reports enable management to quickly identify critical business information such as bottlenecks, performance issues and project delays.
The ability to identify trends across similar projects enables organisations to follow-up on recurring performance issues and continually improve processes, and most importantly, the data that drives these reports is automatically captured through the running of projects in BLUE. This means it is no longer necessary to track data manually in Excel spreadsheets to create meaningful and accurate reports.
BLUE features a number of standard, commonly used KPI reports, which can be expanded upon through using the Data Library that also comes with the product.
Collaborative online proofing solutions
Brand teams comprise decision-makers through sometimes numerous departments throughout the world, as well as vendor partners. This can make collaborating on artwork and copy a lengthily and expensive process. The BLUE™ Online Proofing Module dramatically increases the efficiency in this area, offering a variety of means to compare artwork in formats such as video, print and online.
This versatile software can achieve analysis of artwork across all file types, proving particularly effective for video mode. As a result of these capabilities, previously complex collaborations are simplified and products can be introduced more quickly and economically to the market.
Copy management of websites
BLUE modules utilise XML technology to enable the accurate and efficient publishing of product copy and artwork on packaging, websites and marketing materials. The solution saves each version at every stage of the decision-making chain.
In addition, the finished version is protected and can be quickly distributed to multiple parties through the platform.
The BLUE Copy Management Module boosts the speed-to-market and significantly reduces errors that can occur in copy management systems that are document-based. Copy and artwork can be worked on through the innovative interface, enabling a full audit trail of collective distribution, revisions and approval.
Software for cohesive implementation of product development cycles
BLUE Software’s team are able to provide a system implementation lifecycle (SILC) that is fully documented. Each system roll-out is performed with a process that is clearly defined and time-tested, with monitoring and controls for schedule, budget, quality and scope. The BLUE team uses a collaborative approach to successfully implement its solutions.
BLUE’s team takes care of implementation management of a client’s product, liaising with the relevant project managers to ensure that the development process is sufficiently resourced. Project management by BLUE focuses on closely monitoring, controlling the four key areas of scope, schedule, budget, and quality.
Each project stage is clearly defined with what is required and when it will be delivered.
Quality management system and validation of branded cosmetic products
BLUE is used by many pharmaceutical and medical device companies with extensive regulatory requirements relating to electronic records and signatures.
The BLUE team has an established Quality Management System (QMS) developed to replicate similar quality standards such as ISO and cGMP. The QMS covers system development lifecycle, implementation timeline and system upgrade schedule. It incorporates all operations functions, objectives and activities that contribute to delivering products and services of a consistent quality.
BLUE lifecycles are structured frameworks for maintaining traceability between all phases and providing discipline throughout all aspects of product development and delivery. These internal processes ensure delivery is achieved to all clients, with products well-tested and supported.
About BLUE software
BLUE software is used globally by some of the largest corporations in the pharmaceutical, medical device and consumer goods industries. Since 1997, BLUE’s team has provided its comprehensive expertise in managing graphics and copy according to industry regulations.
To save time in the implementation process, a BLUE validation package can be provided to ensure a quicker time-to-market and that industrial requirements are adhered to.
Products and Services
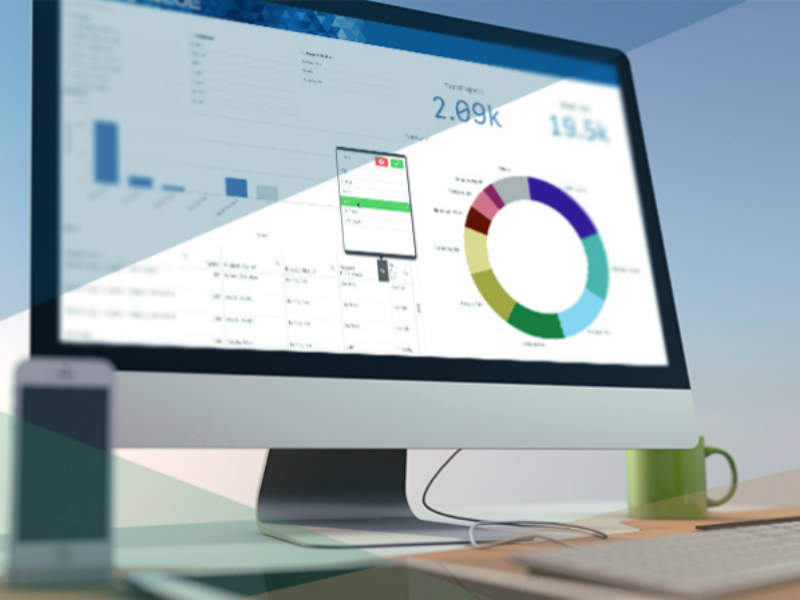
BLUE Business Intelligence

BLUE Software Label and Artwork Management
BLUE Software helps simplify the development and deployment of package labelling and artwork.

BLUE Workflow Management
The BLUE Workflow Management service supports creative, brand, and regulatory stakeholders to manage the label and artwork process.

BLUE Digital Asset Manager
BLUE Digital Asset Manager allows for efficient storage, management, and distribution for large numbers of digital assets across multiple channels.

BLUE Online Proofing
With the BLUE Online Proofing service, a large number of people can simultaneously view, compare, and annotate artwork for print, video, and online deployments.
Video
Related Projects
Press Release
BLUE Software has announced it will be attending two key events this year, GS1 Connect and Pharma and Device Packaging and Labelling.
Read moreBLUE Software to release new software as a service (SaaS) products to its label and artwork management (LAM) technology to mark the company's 20th anniversary.
Read moreRegional Offices
8430 W. Bryn Mawr Avenue
Suite 1100
Chicago
60631
Illinois
United States of America